Abstract
Background
Adenosine is a major signaling nucleoside that activates cellular signaling pathways through a family of four different G protein-coupled adenosine receptors (ARs), A 1, A 2A, A 2B, and A 3. At steady state conditions, extracellular levels of adenosine remain low (10 to 200 nM) either through its rapid cellular uptake by specialized nucleoside transporters, mainly through the equilibrative nucleoside transporter 1 (ENT1), or its degradation by adenosine deaminases. However, the extracellular levels of adenosine can be rapidly elevated up to 100 μM in response to hypoxia, inflammation, or tissue injury. Under pathophysiological conditions, adenosine signaling is involved in modulating inflammation, fibrosis, and ischemic tissue injury. In sickle cell disease (SCD), adenosine signaling is enhanced and contributes to the pathophysiology of the disease. Despite the importance of adenosine signaling in regulating cell proliferation, and stem cell regeneration, as well as in red blood cell functions and adaptation to hypoxia, very little is known about its implication in hematopoiesis, and more specifically during erythropoiesis. Here, we aimed to investigate the effects of high extracellular adenosine on the erythroid commitment and differentiation of hematopoietic progenitors, and to decipher the implication of ARs in these processes.
Results
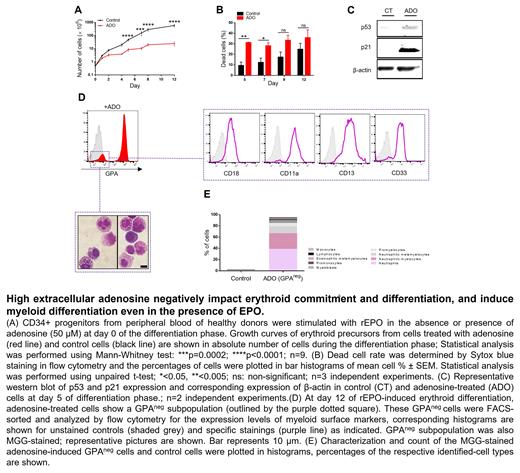
To investigate the role of high extracellular adenosine in regulating erythroid commitment and differentiation of hematopoietic progenitors, we performed ex vivo erythropoiesis of healthy CD34 + cells in the presence or absence of increased extracellular adenosine concentrations ranging from 10 to 200 µM. Our results showed that adenosine decreases erythroid proliferation in a dose dependent manner. High adenosine levels (>50μM) inhibited the proliferation of erythroid precursors and increased apoptosis via a cell cycle arrest in G1. Accordingly, western blots revealed the accumulation of p53 and its downstream target p21, a well-known mediator of G1 cell-cycle arrest, in adenosine-treated cells.
Moreover, adenosine treatment led to the persistence of a non-erythroid GPA neg subpopulation expressing myeloid markers (CD18, CD11a, CD13, CD33). May-Grünwald Giemsa staining of this subset revealed granular cells at different stages of differentiation. The culture of FACS-sorted CD36 + and CD36 - cells suggested that this adenosine-induced GPA neg population originates from the survival of CD36 - myeloid progenitors even in the presence of erythropoietin. Importantly, these effects were specific to adenosine as neither guanosine, uridine nor cytidine affected the proliferation and differentiation of erythroid precursors. Furthermore, we have recently shown that ENT1-mediated adenosine uptake is essential for optimal erythroid differentiation. Therefore, we suggested that elevated extracellular adenosine perturbs erythropoiesis via its signaling upon ARs activation. To confirm this hypothesis, we assessed the effect of ARs activation during erythropoiesis. Given that A 2B and A 3 are the only known ARs expressed in human hematopoietic progenitors and erythroid precursors, we used BAY60-6583 and CI-IB-MECA, two highly selective agonists for A 2B and A 3 receptors, respectively. Both BAY60-6583 and CI-IB-MECA increased apoptosis and decreased erythroblast maturation and enucleation, while only Cl-IB-MECA led to the upregulation of CD33 and CD11a myeloid markers and promoted the differentiation of a GPA neg myeloid subpopulation.
Conclusion
Overall, our results place adenosine signaling as a new player in hematopoiesis regulation. Adenosine signaling via A 3 perturbs erythropoiesis and promotes the survival and differentiation of myeloid progenitors even in an erythroid favoring environment. While the activation of A 2B hampers optimal erythropoiesis without impacting the myeloid differentiation. As both ineffective erythropoiesis and increased leucocyte counts are reported in SCD, and given the detrimental role of high adenosine levels in its pathophysiology, further studies are ongoing to address the impact of adenosine signaling on hematopoiesis in this disease.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal